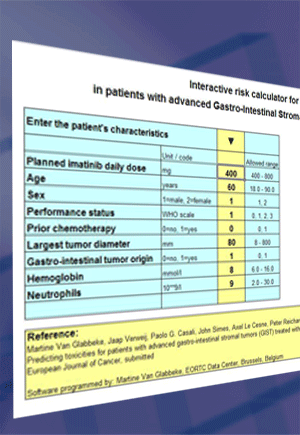
 |
The provided software implements the risk models published in the paper:
Martine Van Glabbeke, Jaap Verweij, Paolo G. Casali, John Simes, Axel Le Cesne, Peter Reichard, Rolf Issels, Ian R. Judson, Allan T. van Oosterom, Jean-Yves Blay. Predicting toxicities for patients with advanced gastro-intestinal stromal tumors (GIST) treated with imatinib:
a study of the European Organisation for Research and Treatment of
Cancer, the Italian Sacoma Group and the Australian Gastro-Intestinal
Trials Group.
European Journal of Cancer 42 (2006) 2277-2285
It allows the user to estimate the probability of experiencing
- edema (CTC grade 2+),
- lethargy (CTC grade 2+),
- skin rash (CTC grade 2+),
- nausea (CTC grade 2+),
- diarrhea (CTC grade 2+),
- anemia(CTC grade 3+)
- neutropenia (CTC grade 3+)
- for individual GIST patients treated with imatinib mesylate,
at doses ranging from 400 mg/day to 800 mg/day.
Estimations are based on the intended daily dose and 8 factors:
- Age
- Sex
- Performance status at treatment start
- Prior chemotherapy (N/Y)
- Largest diameter of the largest lesion
- Identified gastro-intestinal origin of the tumor (N/Y)
- Hemoglobin level at treatment start
- Neutrophil count at treatment start
Version available as Excel spread sheet
Compact version available for handheld computers
Software programmed by Martine Van Glabbeke,
EORTC Data Center.
Download the calculator |

